Initial triage of all emergency presentations involves assessing the airway, breathing and circulation.
This article will look at these first three steps (A = airway; B = breathing; C = circulation) and the more common conditions that cause dysfunction in these body systems.
Emergency presentation of small animal patients can range from acute traumatic incidents to decompensation of a chronic condition. Prompt triage is vital in the management of such conditions because it allows rapid stabilisation.
This article will focus on the most common emergency situations identified during triage. These cases can be both incredibly challenging and highly rewarding for all members of the veterinary team.
In general, staff should obtain as much information about the patient’s signalment, clinical signs and history prior to them arriving – this allows the team to prepare the necessary equipment. A coordinated team approach to information gathering, while a focused examination is taking place, allows for more rapid initiation of treatment.
It is always critical to perform as much of a physical exam as reasonably possible prior to administering therapies, as this will guide treatment, monitoring and prognosis. However, analgesia should never be withheld.
Primary survey should be performed within minutes of the patient’s arrival and should be quick to do; airway, breathing and circulation are all assessed immediately. This should be followed by a more thorough investigation of the major body systems (respiratory, cardiovascular and neurological) to guide further investigation or therapeutics. Abnormalities identified at either of these two points should be addressed, and the patient stabilised, before progressing to further diagnostic procedures.
It is also worthwhile to apply this same process in any hospitalised patient when their clinical condition deteriorates while undergoing treatment.
Airway
Initial assessment
Any dyspnoeic patient should be handled carefully and efficiently to minimise oxygen consumption. Supplemental oxygen therapy can be provided while an initial physical exam is performed.
When assessing the airway, check:
- Is the airway clear?
- foreign material – for example, ball or stick
- bodily fluids – for example, vomit
- Are signs of upper airway obstruction present?
- increased inspiratory effort
- stridor – for example, laryngeal paralysis
- Is this a large-breed, older dog?
- stertor – brachycephalic obstructive airway syndrome; nasopharyngeal polyps in young cats
While pattern recognition is generally discouraged, in these emergent cases it can help to direct diagnostic and therapeutic plans efficiently – particularly for localising the source of respiratory distress.
Initial management
Supplemental oxygen therapy should be instituted initially. Additionally to this, sedation can be used to ease anxiety1.
Typically, an opioid sedative – such as butorphanol (0.2mg/kg to 0.4mg/kg) – is useful in most cases where potent analgesia is not required. Low-dose acepromazine (0.01mg/kg to 0.02mg/kg) may be required for additional sedation, as long as the circulatory system is not compromised.
In cases of imminent respiratory failure, intubation and intermittent positive pressure ventilation may be required to take control of ventilation. In cases where impending respiratory fatigue and marked respiratory distress are evident, induction of anaesthesia and intubation will often be made based on clinical judgement, as blood sampling may increase stress.
In other situations, the decision is made based on blood gas analysis: severe hypoxaemia (partial pressure of oxygen less than 60mmHg) indicating poor oxygenation despite oxygen supplementation, or severe hypercapnia (partial pressure of carbon dioxide greater than 60mmHg) indicating decreased ventilation.
An example of such a patient would be a French bulldog presenting tachypnoeic and dyspnoeic with cyanotic mucous membranes showing signs of upper airway obstruction secondary to brachycephalic obstructive airway syndrome. Such patients are incredibly fragile and require immediate sedation to decrease their anxiety and work of breathing; as well as reducing oxygen demand, reducing the work of breathing should reduce further inflammation of the upper airways.
In such patients (Figure 1), rapid intubation and securing of their airway with an endotracheal tube allows for diagnostics to be performed while allowing time for airway swelling to reduce.
Tracheostomy is rarely required in an emergency and ideally approached in a sterile controlled manner, although the equipment and skills necessary to deal with “a difficult airway” should always be prepared prior to attempting intubation of these patients.
Management of hyperthermia is vital and often overlooked. Cooling measures – such as fans, spraying the patient with tepid water and IV fluids – can be easily implemented at the same time as other emergency treatments. Cold/icy water should be avoided as this may lead to vasoconstriction in the periphery and hinder the cooling process.
Typically, brachycephalic patients with severe upper respiratory tract obstruction will be hyperthermic due to impaired heat loss. Body temperature higher than 41.6°C significantly increases tissue oxygen consumption, and can lead to disseminated intravascular coagulation and multiple organ failure2.
Temperature should be closely monitored and active cooling should be stopped when body temperature reaches approximately 39.4°C.
When providing oxygen therapy using a tent or cage, the humidity and temperature of these environments can rise rapidly and exacerbate respiratory distress.
Breathing
Assessing breathing
When assessing breathing:
- Increased respiratory drive, be that physiological or pathological, will result in recruitment of the secondary muscles of respiration. Evidence of paradoxical or uncoordinated respiration can indicate respiratory distress.
- Posture and facial expression – dyspnoeic patients will often abduct their elbows, extend their necks and breathe through an open mouth to minimise resistance to airflow.
- Thoracic auscultation – examples of findings are:
- dull/quiet lung sounds:
- “something in the way” – such as pneumothorax, pleural effusion or diaphragmatic hernia
- collapsed lung/atelectasis – pneumonia, abscess, pulmonary neoplasia
- crackles:
- for example, pneumonia, contusions, coagulopathy
- fibrosis – for example, West Highland white terrier idiopathic pulmonary fibrosis (Video 1)
- pulmonary oedema (Figure 2):
- cardiogenic
- non‑cardiogenic pulmonary oedema – for example, electrocution, suffocation, strangulation, acute respiratory distress syndrome
- wheezes:
- lower airway collapse or constriction – typically causes increased expiratory effort:
- feline lower airway disease
- chronic bronchitis
- lower airway collapse or constriction – typically causes increased expiratory effort:
- dull/quiet lung sounds:
Initial management
Oxygen therapy should be implemented immediately; individual patient tolerance to this may vary (Table 1). Some patients will tolerate flow-by oxygen, while others will resent this technique. Such patients often require sedation, gentle handling and oxygen supplementation in an oxygen tent (Figure 3).
Investigation
Point-of-care ultrasound of the thorax (Figure 4) is a rapid, minimally invasive diagnostic tool that is gaining popularity in emergency management. It can be used to quickly identify pleural effusion, pneumothorax, fluid within the lungs (blood, water), pericardial effusion, subjective cardiac contractility and cardiac chamber enlargement.
This form of imaging has been shown to aid in differentiating cardiogenic respiratory disease from non-cardiogenic respiratory disease in most cases in cats4. It is also useful for this purpose in dogs, although, typically, less difficulty exists in differentiating cardiac and non-cardiac-related dyspnoea based on history and clinical exam compared to cats5.
Where pulmonary oedema is identified in conjunction with evidence of congestive heart failure, diuretic therapy can be started. The most commonly used first-line diuretic is furosemide, typically used at an initial dose of 2mg/kg to 4mg/kg, given as a bolus IV if possible, or IM if IV access cannot be obtained.



Management
Thoracentesis (Figure 5) is one of the few procedures in emergency medicine that is both diagnostic and therapeutic.
In cases of severe pleural space disease, thoracentesis is likely to be the only way to resolve the respiratory compromise. Prior to doing this, relevant history – including access to anticoagulant rodenticides – should be obtained.
If haemothorax secondary to rodenticide toxicity or other coagulopathy is suspected, thoracentesis should only be performed if it is required to relieve respiratory distress.
Avoid removing an excessive volume, as the remaining blood in the thorax will be resorbed into circulation6.
Autotransfusion can be considered if significant hypovolaemic/hypoxaemic shock is evident.
A history of trauma (particularly blunt trauma to the thorax) should always raise concern for the possibility of pneumothorax. Cases of tension pneumothorax will need urgent thoracentesis.
Respiratory compromise may not be evident initially, but can develop gradually. A recent study of 364 dogs suffering thoracic trauma found a 12% prevalence of lung lacerations, with 25% of those cases developing pneumothorax7. Progression or development of pulmonary contusions in the initial 24-hour to 36-hour period following trauma can also lead to respiratory compromise.
In patients showing evidence of septic shock, or with evidence of bacterial involvement, prompt antimicrobial therapy is recommended.
One study comparing thoracic point‑of‑care ultrasound findings to chest radiographs in cases of respiratory disease identified that pneumonia more commonly resulted in alveolar-interstitial syndrome (AIS) in the right unilateral thorax or, less commonly, in the left cranial thorax; whereas cardiogenic AIS was typically a diffuse or a bilaterally caudal pattern8.
While it is important to note these patterns are not consistent across all cases, it can aid decision‑making in emergency situations or where access to advanced imaging – such as CT – is not available, or where the patient is not stable enough for procedures such as bronchoscopy and bronchoalveolar lavage.
Temperature is also helpful in differentiating non-cardiogenic and cardiogenic respiratory distress in cats. The RAPID cat study9 found acutely dyspnoeic cats with no signs of trauma, presenting with a rectal temperature below 37.5°C or a pulse greater than 200bpm, or a respiratory rate greater than 80 breaths per minute, were significantly more likely to be in congestive heart failure.
An example of such a case would be a 14‑year‑old cat presented tachypnoeic with shallow breaths, reduced lung sounds bilaterally on auscultation and open-mouth breathing when handled. Feline patients are particularly susceptible to stress during handling and often benefit from sedation with an opioid – such as butorphanol – to reduce anxiety, and placement in an oxygen tent as an initial step.
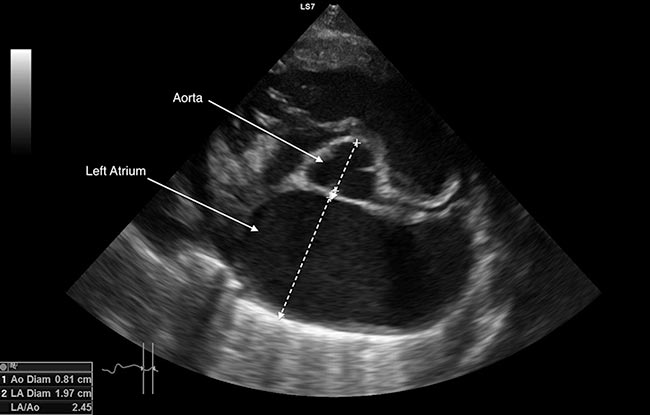
A point-of-care ultrasound scan can be used to confirm the presence of pleural effusion, and a left atrial to aortic diameter ratio (LA:Ao) can be used to ascertain if congestive heart failure is the underlying cause (Figure 6).
An LA:Ao greater than 1.5 is consistent with cardiac disease4. LA:Ao can, however, be very difficult to obtain in dyspnoeic cats4.
The left atrial diameter on the long‑axis right parasternal view can be used when it’s not possible to obtain a reliable LA:Ao. A left atrial diameter greater than 1.65cm in conjunction with signs of congestion – such as B lines, pleural effusion or pericardial effusion – is consistent with congestive heart failure10.
Circulation
Initial assessment
A state of shock needs to be rapidly identified and addressed. Assessment of the circulatory system involves examination of mucous membrane colour, capillary refill time (CRT), heart rate and rhythm, pulse pressures and temperature.
This article focuses on the various forms of shock, how to differentiate them and the first stages of treatment.
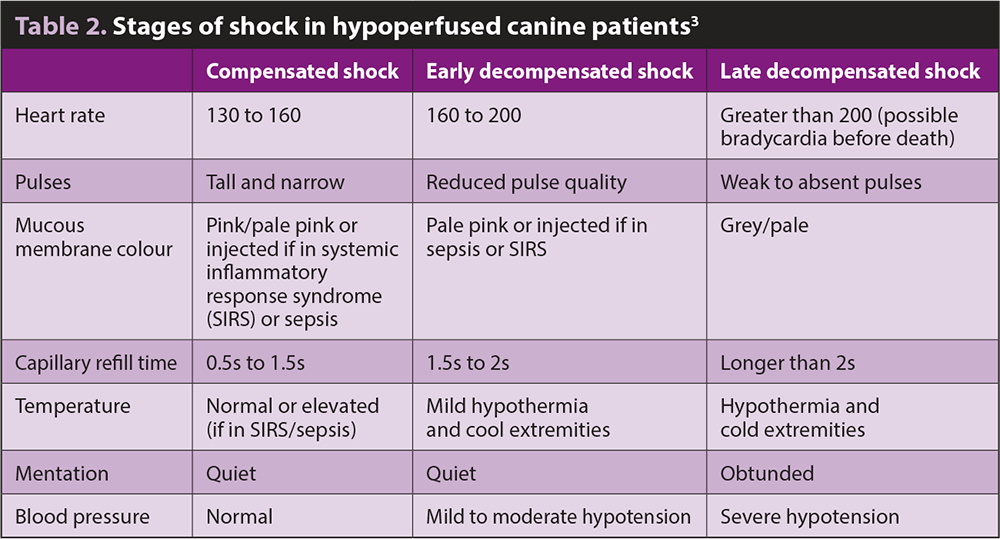
Shock is, essentially, a disruption in cellular energy production due to oxygen delivery not meeting cellular demands. This initially starts as a compensated state, whereby the body is able to maintain some level of homeostasis through measures to meet minimum oxygen delivery demands. As demands exceed the coping mechanisms, the products of altered metabolism accumulate, and the body starts to enter a decompensated state (Table 2).
The categories of shock are divided into six main groups to guide therapy:
- Hypoxaemic: reduction in either partial pressure of arterial oxygen (pulmonary disease) or haemoglobin (anaemia) resulting in tissue hypoxia:
- Causes of haemoglobin loss include haemorrhage, haemolysis and reduced bone marrow production:
- clinical signs: tachycardia, pale mucous membranes, normal CRT, tachypnoea
- Causes of decreased oxygen saturation include ventilation/perfusion mismatch, hypoventilation, diffusion impairment, reduced fraction of inspired oxygen and low venous oxygen levels:
- clinical signs: tachycardia, cyanotic mucous membranes, normal CRT, tachypnoea/dyspnoea, strong pulses
- Causes of haemoglobin loss include haemorrhage, haemolysis and reduced bone marrow production:
- Hypovolaemic: reduced circulating blood volume, leading to a reduction in preload, reducing stroke volume – which then reduces cardiac output, causing a reduction in mean arterial pressure and oxygen delivery to tissues, resulting in tissue hypoxia:
- Causes include haemorrhage, severe dehydration, trauma, gastrointestinal losses, and effusions.
- Compensated: mild‑moderately dull mentation, tachycardia, normal blood pressure, normal to prolonged CRT, tachypnoea, cool extremities, normal peripheral pulses.
- Decompensated: dull mentation, tachycardia, hypotension, prolonged CRT, pale mucous membranes, poor pulse quality.
- Obstructive: obstruction to venous return to the heart:
- Causes include gastric dilatation‑volvulus, pericardial effusion, tension pneumothorax and caval syndrome – for example, in heartworm infection.
- Clinical signs are often similar to hypovolaemic shock.
- Distributive: vasodilation leading to venous pooling and reduction in systemic vascular resistance, ultimately causing a drop in mean arterial pressure (MAP) and oxygen delivery, resulting in tissue hypoxia and poor perfusion:
- Causes: sepsis, anaphylaxis and neurogenic factors.
- Hyperdynamic (early) phase – tachycardia, bounding pulses, rapid CRT and hyperaemic mucous membranes.
- Hypodynamic (late) phase – tachycardia, hypotension, pale mucous membranes, poor pulse quality, dull mentation, prolonged CRT.
- Cardiogenic: failure of the heart to maintain forward flow of blood to the arterial system, typically with a normal to increased circulating blood volume. This reduces cardiac output, subsequently leading to reduced MAP and oxygen delivery, resulting in tissue hypoxia:
- Causes include severe valvular disease, myocardial disease, cardiac arrhythmias and, less commonly, toxicities involving anaesthetics.
- Clinical signs include tachycardia, weak pulses, pale or cyanotic mucous membranes, prolonged CRT, cold extremities, tachypnoea and hypothermia.
- Metabolic: the cells are unable to use oxygen and nutrients normally, despite normal tissue perfusion and oxygen delivery:
- Causes include hypoglycaemia, cyanide toxicity and mitochondrial disease.
- Clinical signs: variable, tachycardia, tachypnoea, may have red mucous membranes.
- Cats in shock can either present with tachycardia or bradycardia, and they are more prone to respiratory dysfunction than dogs.
Early recognition of shock is critical, but can be difficult, particularly in the early stages11. Serial physical examination allows early recognition of shock in emergent patients, and allows assessment of response to treatment and return to cardiovascular stability.
The shock index (SI), which calculates a ratio of heart rate to systolic blood pressure, has been proposed as a means of helping to identify patients in shock in combination with other clinical parameters. Currently, limited evidence exists in canine patients for the use of the SI and a dearth of evidence for feline patients. However, it is gaining more attention12.
The SI has been widely used and studied in human literature as a rapid means of identifying early signs of shock. More research needs to be undertaken to be able to reliably use this measure in our veterinary patients; however, it may be a useful and easy clinical tool to help pick up patients in the early stages of hypoperfusion when used cautiously in combination with a thorough physical exam.
One study11 suggested a SI greater than one warrants further investigation for blood loss in patients presenting on an emergency basis.
Investigations
Lactate measurements are commonly used as a marker of anaerobic metabolism associated with reduced tissue oxygen delivery.
Single time point lactate measures are not consistently associated with outcome and can differ greatly depending on the disease process, as well as a patient’s ability to correct hyperlactataemia.
On the other hand, serial lactate measurements and clearance rates are more indicative of survival rates, with persistent hyperlactataemia being associated with high mortality rates. Therefore, making clinical decisions based on a single admission lactate measurement is not recommended13.
Management
The main aim in treating shock is to restore oxygen delivery to tissues. In most cases of shock, this initially involves administration of a large volume of crystalloid fluid (Figure 7). The exceptions to this are cardiogenic shock, patients that cannot tolerate large volumes of fluid and, to some extent, “hypotensive resuscitation” – aiming to maintain a MAP of 65mmHg – in cases of active bleeding (Figure 8).
Crystalloid: 10ml/kg to 30ml/kg boluses over 5 to 15 minutes, with reassessment of patient after each bolus.
Colloid: low oncotic pressure cases (for example, acute hypoalbuminaemia) – increases intravascular volume more rapidly with a smaller volume and helps retain intravascular fluid for longer:
No evidence exists at this stage to show increased risk of acute kidney injury in animals, but the available studies are limited14.
Hypertonic saline 7.2% solution: draws fluid from the intracellular/extravascular space into the intravascular space – transient effect lasting less than 30 minutes. Indicated for rapid improvement in circulating volume to allow other therapies to take effect. Important to also provide other resuscitative fluids to restore intracellular and interstitial fluid.
Blood products: in patients not responsive to fluid therapy, most can tolerate acute haemodilution down to a haematocrit lower than 20%, although concurrent patient problems need to be considered:
packed red blood cells to improve oxygen supply and fresh frozen plasma for patients with coagulopathy
Introduce vasopressor therapy if patients aren’t responding and in vasodilatory shock – for example, septic shock.
Cardiogenic shock treatment: minimise stress, oxygen therapy, diuresis/inotrope, sedation.
Complications of overzealous IV fluid administration include tissue oedema, with the most concerning location being pulmonary oedema as well as dilutional coagulopathy15.



Conclusion
Dealing with emergency cases rapidly and effectively is hugely beneficial to your patients and highly rewarding for staff.
Basic management of emergencies and critical patients should be achievable in all practices with the institution of a triage system and whole-team training. The first three steps of a basic triage are covered in this article, so you can apply them in your practice.
A systematic approach of checking the airway, breathing and circulation in all patients will allow efficient identification and treatment of life-threatening conditions to give you and your team more confidence when handling emergencies.
Common emergency presentations – the initial triage: as easy as ABC







0 Comments